Introduction: Chimerism changes post hematopoietic cell transplant (HCT) can be an early indication of disease relapse. Graft immune cell fraction composition has been seen associated with clinical outcome of hematopoietic cell transplantation patients (Saliba et al., Haematologica February 2020). AlloSeq HCT (CareDx Inc., CA, USA) is the latest innovation in chimerism testing, an NGS-based chimerism assay that uses a targeted SNP panel and provides standardized workflow with automated data analyses.
Objectives: We aimed to analyze graft immune cell composition using the AlloSeq HCT assay and compared it to the current standard laboratory method based on Short Tandem Repeat (STR) analysis.
Methods: A total of 8 post HCT samples from peripheral blood were taken at the same time point for cellular subfraction and isolation of CD15+, CD33+, granulocytes (GR) and monocytes (MN) cells prior to genomic DNA (gDNA) extraction. For each recipient, donor gDNA was input as a reference sample. The kit-based test AlloSeq HCT was used to prepare libraries and sequenced on the Illumina MiSeq platform. AlloSeq Software performed the QC and data analysis for the evaluation of percent chimerism (median ± CI 95%). Results below limit of detection (LOD of 0.32% for AlloSeq HCT and 3% for STR) were excluded in the Mann-Whitney and Spearman correlation analyses. For inter-assay variation 3 subfraction samples with results along the dynamic range were repeated 2-3 times on AlloSeq HCT and the coefficient of variation (CV) was calculated.
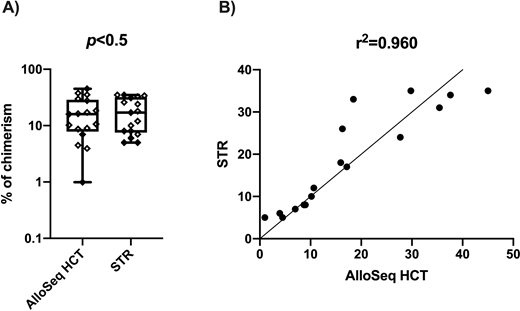
Results: CD3+ cells dominated graft cellular composition with a chimerism of 31.6% (10.4 to 44.3%), followed by MN cells at 10.0% (1.4 to 18.6%) and GR cells at 7.3% (0.56 to 13.9%), and CD15+ was the smallest fraction with 2.9% (-4.0 to 9.8%). Median levels of chimerism in each cellular subfraction of the grafts were not statistically significant different when comparing AlloSeq HCT and STR (p>0.5) (Fig. 1A). Results of percent of chimerism obtained for each cell fraction using both methods exhibited an overall correlation of r=0.96 (CI 95%: 0.88 to 0.98) (Fig. 1B). LOD results were reported in 5 with AlloSeq HCT compared to 9 with STR out of the total 26 subfraction tested (19% vs 35%). The inter-run CV was 1.7, 14.5 and 1.6 with a means percent chimerism of 0.8, 17.0 and 14.3, respectively.
Conclusion: The immune cell composition of the graft can be characterized in clinical practice with NGS-based chimerism assay. Evaluation of AlloSeq HCT showed excellent assay performance and a larger dynamic range compared with STR, as well as increased sensitivity by reporting 4 cases where percent chimerism was undetectable by STR. NGS-based medicine shows great promise for better detection and definition of HCT graft with a streamlined workflow.
Kyle:CareDx: Current Employment. Casas:CareDx: Current Employment. Grskovic:CareDx: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties, Research Funding. Viard:CareDx: Current Employment, Current equity holder in publicly-traded company, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal